Precipitation of Aluminum Hydroxide From Aqueous Solution
Solution tank aluminum hydroxide precipitation particles Prior art date 1951-09-22 Legal status The legal status is an assumption and is not a legal conclusion. Write the net ionic equation for the precipitation of calcium hydroxide from aqueous solution.

Solved Aqueous Solutions Of Aluminum Chloride And Sodium Hydroxide Are Mixed Forming The Precipitate Aluminum Hydroxide
Aqueous solutions of strontium bromide and aluminum nitrate are mixed.

. Aqueous solutions of rubidium hydroxide and cobaltII chloride are mixed. The precipitation of aluminum from AICh solutions was found to be complete at sub. A colloidal aluminum hydroxide hydrosol formed in acid solutions up to hydroxidealuminum ratios of 30 and a settleable precipitate formed between 30 and 40.
More 40 wt. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitateWhether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. The chemical behavior of aluminum in dilute solutions presents some serious experimental difficulties.
SUMMARY Aluminum oxide has been prepared by thermal dissociation of alu- minum hydroxide obtained by precipitation with formalin from sodium. Aluminate mother liquor is passed countercurrent to the direction of feed of aluminum hydroxide crystals through at least two stages each of which has a lower turbulent zone with a high suspension density and which is agitated under conditions which assure particle growth via crystal growth and an upper non-turbulent stagnant liquid layer from which most crystals. The coprecipitations were monitored by potentiometric pH titration and the.
The principal aluminum species detected in acid solutions by computer analysis of. Once the procedure is completed waste removal is carried out leaving the solution of sodium aluminate to precipitate. And amorphous AlOHgc A colloidal aluminum hydroxide hydrosol formed in acid solutions up to hydroxidealuminum ratios of 30 and a settleable precipitate formed between 30 and 40.
Up to 10 cash back As the pH rises the aluminum salt is hydrolyzed to form aluminum hydroxide. The precipitation of aluminium hydroxide is promoted by bubbling carbon dioxide gas into the supersaturated sodium aluminate solution ie the carbonation process Misra 1986 Zhao et al 2004 meanwhile the caustic in the sodium aluminate solution is converted to sodium carbonate which is recycled to extract alumina from the bauxite in the sintering process. A precipitation chemical reaction has.
In turn aluminum hydroxide absorbs organic pollution dyes surfactants inorganic ions 4 5. Most reactions involving aluminum and hydroxide proceed slowly. The precipitation and crystallization of aluminum oxyhydroxides from chloride solutions by neutralization appears to be quite complex and depends on several parameters namely temperature the OHAl molar ratio aging and solution composition.
It is important to realize that this equilibrium is established in any aqueous solution containing Ca 2 and CO 3 2. Calculate the solubility of aluminum hydroxide AlOH 3 in a solution buffered at pH 1100. The process involves first a nucleation stage to form primary seeds by adding an acidic aluminium sulphate solution to a Bayer plant liquor which contains 210 gL.
Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed Expired - Lifetime Application number US247861A Inventor Ralph W Brown. By adding a 40 wt NaOH solution aluminum was removed from the wastewater as a hydroxide by precipitation particles of 10-90 lm are expected Andersson and Hansson 2001. Solid leadII acetate is added to an aqueous solution of ammonium iodide.
Standard free-energy values for the common solid forms of aluminum hydroxide also disagree. Magnesium aluminium hydroxides were coprecipitated from different mixed metal cation solutions at total CM 01 M and MgAl2ratios from 1 to 6 with sodium hydroxide solution at ambient temperature with different pre-ageing conditions for the aluminium hydroxide pre-precipitate. In aqueous solution mathAl3math is present as the.
It is possible to decant the supernatant solution comprising mathCr O_42-math from the solid mathAlOH_3math identified as the white gelatinous precipitate. The precipitation of fine aluminium hydroxide Al OH 3 powders average particle size 2 μm from Bayer plant liquor was investigated which is important for the production of specialty alumina Al 2 O 3 products. Aqueous solutions of barium chloride and lithium sulfate are mixed.
When we add excess sodium hydroxide to aluminium sodium aluminate is given. For the subsequent extraction of the dispersed phase various methods namely. We would place a few drops of the NaCl solution in the reaction container followed by a few drops of AgNO 3 solution and observe an immediate cloudiness that indicates a solid precipitate has formed.
Write the net ionic equation for the precipitation of iron III sulfide from aqueous solution. Write the net ionic equation for the precipitation of aluminum phosphate from aqueous solution. The mineral obtained through the.
This problem has been solved. The principal aluminum species detected in acid solutions by computer analysis of potentiometric data were AlOHand Al8OH2j. But adding dilute HCl acid will neutralize OH-ions and will give AlOH 3 white precipitate.
Then the solution is rendered basic enough to make the aluminium precipitate in the form of its hydroxide salt. Aluminium hydroxide is soluble in aqueous sodium hydroxide solution. Predict whether CaHPO 4 will precipitate from a solution with Ca 2 00001 M and 0001 M.
The process involves dissolving bauxite in a solution of sodium hydroxide at 270 degree Celsius. Aluminium hydroxide is a insoluble hydroxide in water. Filtration sedimentation 6 flotation 7 8 electrocoagulation electroflotation 9.
What are the similar reactions to aluminium and. Because not all aqueous reactions form precipitates one must consult the solubility rules before determining. Reaction and net ionic equation.
Let us consider the possible reaction of aqueous solution of NaCl with aqueous solution of AgNO 3.
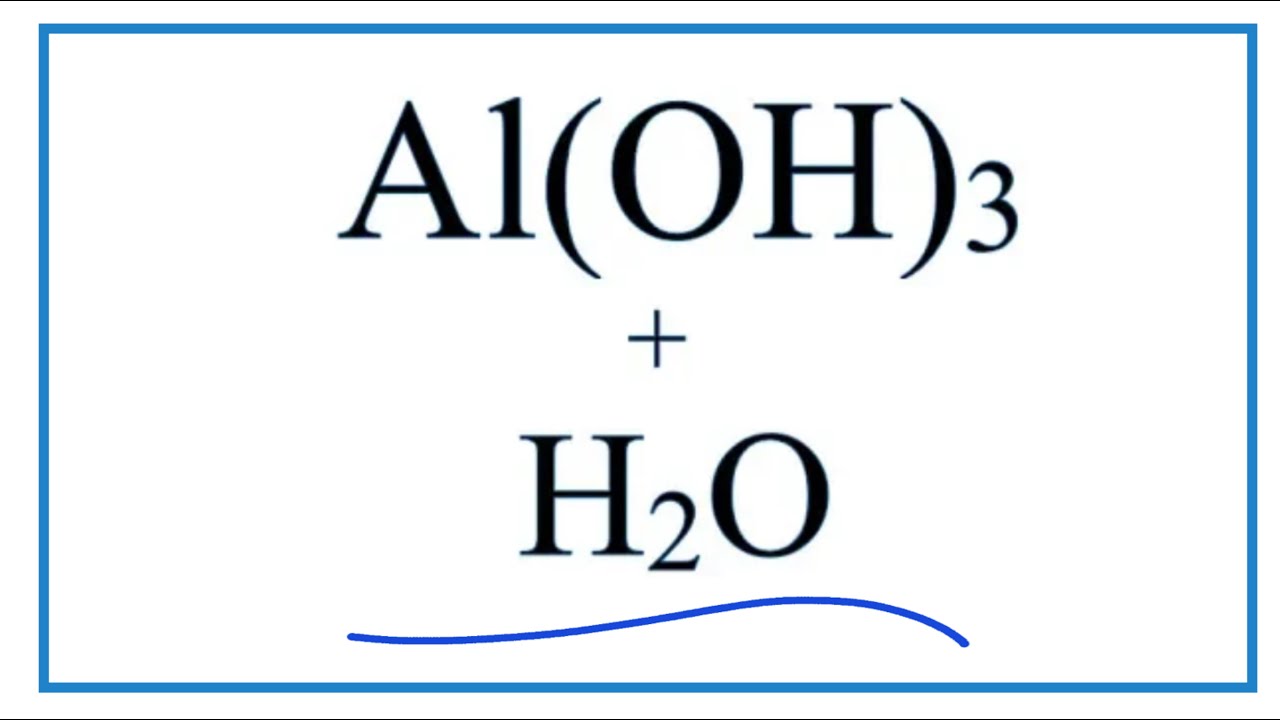
Equation For Al Oh 3 H2o Aluminum Hydroxide Water Youtube
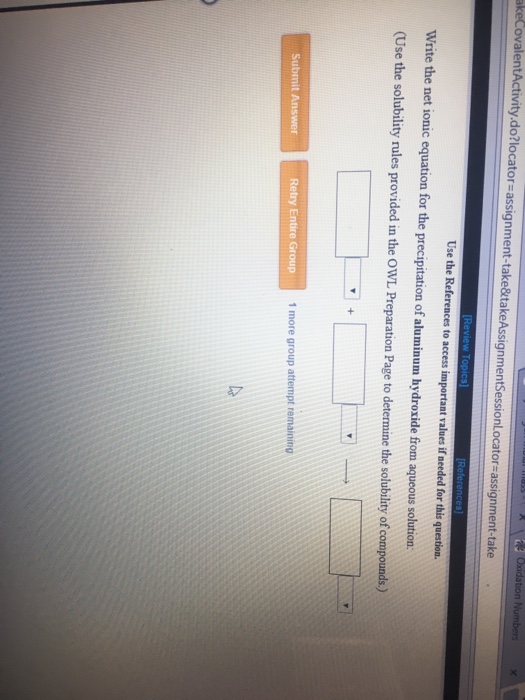
Solved Write The Net Ionic Equation For The Precipitation Of Chegg Com

Solubility Diagram Of Aluminium Hydroxide Al Oh 3 S Considering Only Download Scientific Diagram

Types Of Chemical Reactions Chemistry Education Chemical Reactions Chemistry Class
0 Response to "Precipitation of Aluminum Hydroxide From Aqueous Solution"
Post a Comment